|
 |
|
|
Live Links: To visit the actual site framed, in full-screen, or download the actual document referenced, click anywhere inside the image. |
|
|
|

|
|
Full Membership:
The top 90% of the industry are signed members of these organizations and their guidelines. |
|
|
|
|
|
 |
|
Janssen:
Janssen pharmaceuticals is a signed member of ABPI and The BHBIA. |
|
|
 |
|
These guidelines were found on the first page of results with the simple search string "Clinical Trials Guidelines". An identiccal search on the AMA web site returned 136 documents. None of them were actual guidelines, rather reports from various meetings held by doctors. |
|
 |
|
The Image at left is a sample.
The identical real agreement is a simple, straight forward 30 page document that governs all aspects of clinical trials.
Click on the image to see the whole genuine document
In fact, the portion that details how adverse events are handled is only 3 pages long. (pictured and linked below, left)
|
|
 |
|
Image:
The compensation for adverse events portion (left) is even separated out from the whole agreement and published as its own document on their website.
Click on the image to see the entire genuine document.
|
|
| |
|
|
|
| |
|
|
|
So, that is how Britain, Ireland, Scotland and Wales manages clinical trials. We found nearly identical guidelines and procedures for Canada, Australia and New Zealand. We will update you as we research other countries.
Now The USA, is another matter. |
In the U.S.,when a patient in a clinical trial held by Janssen pharmaceutical has an adverse event, Janssen is under no obligation to even provide the patient with information about the drug that ruined their life, let alone provide compensation or medical expertise.
If the patient needs any sort of information, they must file a report with the FDA, wait up to six months, then file another report with Janssen and wait for the information.
If the patient needs any sort of compensation due to medical costs, loss of income or suffering, their only recourse is ask the FDA and the Center for Drug Evaluation and Research Division of Scientific Investigation, to start an investigation.
The patient must wait 180 days for an investigation to begin.
If they do choose to investigate, the patient must then prove somehow that the drug was the cause of the event.
If somehow they do prove the drug caused the event, it is 100% upon the sick or dying patient to source an attorney, convince them of the financial viability of a law suit, then wait as up to seven years to see if the suit is successful or settled.
|
Why the huge difference in how the same situation is handled in 2 countries who are considered "sister" countries?
|
_________________________In Britain_____________________
This agreement was sponsored by and signed by each member of the (BHBIA) and ABPI, including Janssen Pharmaceutical, because the medical associations and the government ASKED THEM TO. |
_________________________In The U.S._____________________
Neither the AMA nor any elected government representative has, or will ask for the same dignity and safety for it's citizen patients as is universally done not only in Britain, but in most other countries of the world.
|
In particular, we are focusing on an adverse event that occurred in 2008 during a clinical trial of Janssen's now $1.4 Billion blockbuster drug Stelara.
|
 |
|
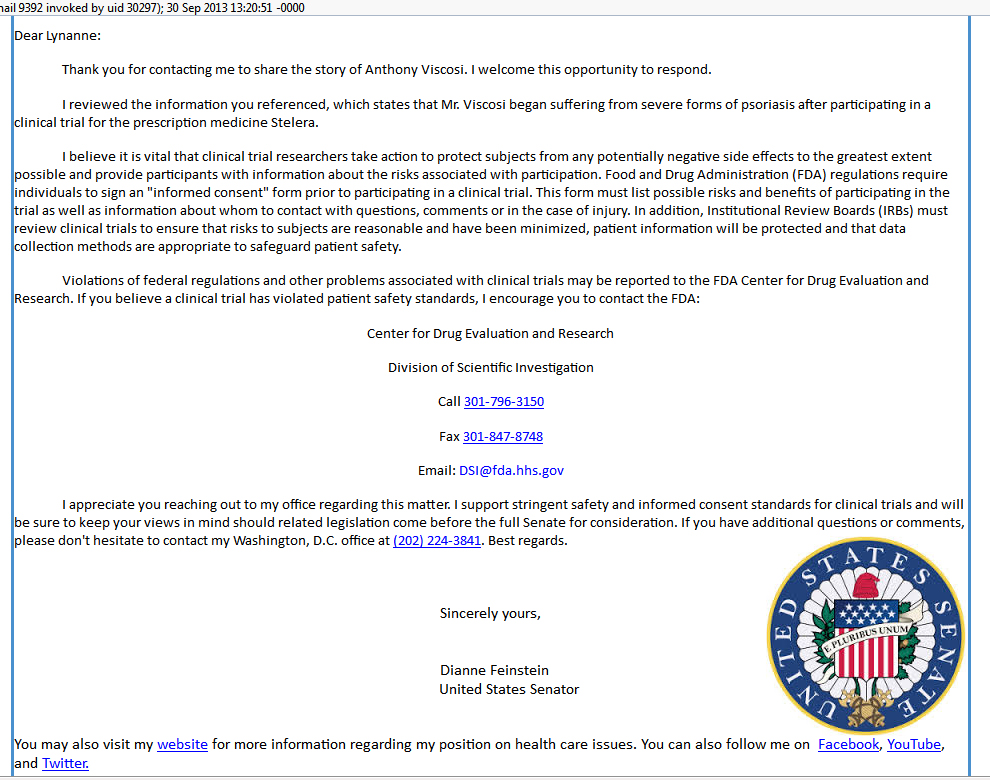
This is a real life response from Dianne Feinstein of California, written to a supporter of a US citizen who suffered a severe adverse event during a clinical trial of CNTO-1275, Now called Stelara, Janssen's $1.5 Billion /year blockbuster drug.
A typical response from a US senator when a US citizen along with 200 supporters, wrote letters to the senator's office and provided a petition with 5000 signatures, including 900 signatures from the senator's own state. |
|
| |
| |
| |
|
|
|
|