|
|


|
|
|
It's January, 2026.
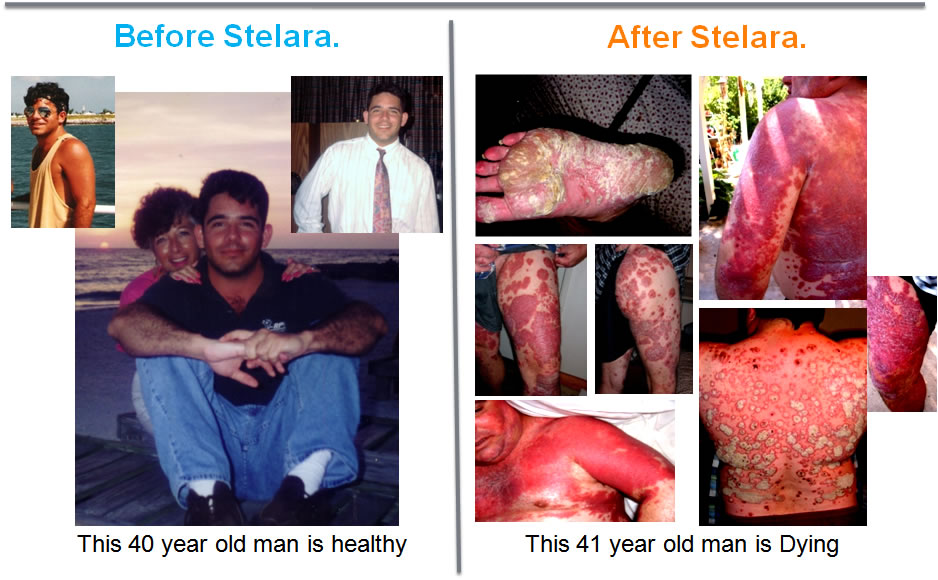
In 2008, this man was prescribed a "New Miracle Drug".
Ever since, he has been suffering, While
the US Government
and Johnson & Johnson stood by, benefitting from the $7 billion in annual sales of toxic biologic drugs.
Despite years of clinical trials evidence that show disasterous, long term effects and new immune damage caused by biologic drug use, the FDA APPROVED the drug which did this to a healthy man.
A man who had only mild Psoriasis for over 7 years.
He then took part in a Clinical Trial of Stelara to treat his mild psoriasis.
A Biologic is any drug who's chemical name ends with MAB, which is an acronym for Mono-Clonal Antibody. |
If you or someone you know has an autoimmune disease,
(Rheumatoid Arthritis, Psoriatic Arthritis, Ankylosing Spondylitis, Dermatomyositis.
Psoriasis, Vitiligo, Alopecia Areata, Scleroderma, Eczema, Celiac Disease, Crohn's Disease, Ulcerative Colitis)
Start here / Do your homework / It may save your life.
#1 -The printable, chronological PDF full story
|
|
|
PREFER VIDEO? Watch the HELP FOR TONY - VIDEO to see the story on YouTube.
|
SME Statements
| 'One mistake was to give the most powerful psoriatic drug ever made to someone who had very mild psoriasis' - PK, PMP |
'The Doctors running this trial certified that this patient had no other types of psoriasis by allowing him in the trial at all. A Documented Exclusion for this Clinical Trial: "Any non-plaque psoriasis" is that certification. If any other psoriasis was present, the Doctors would have disqualified him.' class="RED3">- EV, PI |
'Without specific clinical data, that only the drug's developers can provide, there is little hope that an effective treatment plan can be developed' - BO, MD |
|
| |
|